The raisin pudding model of the atom (j. j. thomson) thomson recognized one of the consequences of the discovery of the electron. because matter is electrically neutral, there must be a positively charged particle that balances the negative charge on the electrons in an atom. Huge sale on j j thomson now on. hurry limited offer. save now!. Thomson atomic model was proposed by william thomson in the year 1900. this j j thomson atom model model explained the description of an inner structure of the atom theoretically. it was strongly supported by sir joseph thomson, who had discovered the electron earlier. during cathode ray tube experiment, a negatively charged particle was discovered by j. j. thomson. More j j thomson atom model images.
Bunyi teori atom j. j. thomson. atom merupakan sebuah bola yang bermuatan positif dengan adanya elektron bermuatan negatif yang berada disekitarnya. muatan positif atau negatif pada atom besarnya sama. hal ini menunjukan atom bermuatan netral. suatu atom tidak mempunyai muatan positif dan muatan negatif yang berlebihan. Jan 19, 2016 thompson's experiments: sir joseph john thomson (aka. j. j. thompson) was an english physicist and the cavendish professor of physics at the . J. j. thomson's model of an atom 1. cathode ray tube. the cathode ray tube (crt) is a vacuum tube containing one or more electron guns (a source of electrons or electron emitter) and a fluorescent screen used to view images. it has a means to accelerate and deflect the electron beam(s) onto the screen to create the images. Find the best deals for atom models. compare prices online and save today!.
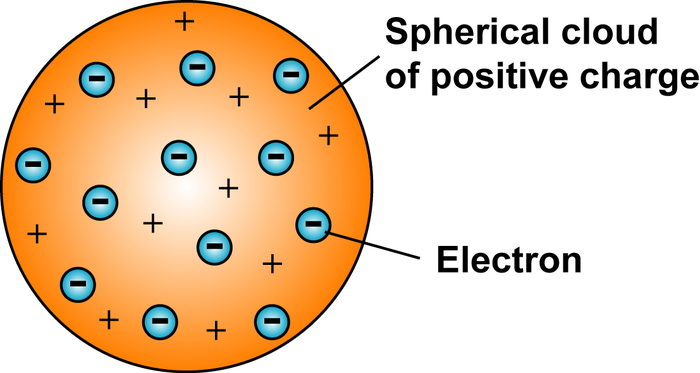
The rutherford model supplanted the “plum-pudding” atomic model of english physicist sir j. j. thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,…. Atom models. j. j. thomson’s model (water melon model) in this model, the atoms are visualized as homogeneous spheres which contain uniform distribution of positively charged particles (figure 8. 8 (a. the negatively charged particles known as electrons are embedded in it like seeds in water melon as shown in figure 8. 8 (b). 1. gross features of the atom j. j. thompson’s experiment. 2 bohr’s model of the atom, 3. outline description of the rutherford’s alpha scattering experiment. period 1: j. j. thompson’s cathode rays experiment. experiments on discharge tubes performed by j. j thomson (1897) led to the discovery of the electron (cathode ray) as a. Thomson's atomic model was proposed in 1904 and was called the plum pudding model. it was introduced right after thomson's 1897 discovery of the electron, .
J J Thomsons Plumpudding Atomic Model The Making Of A
Thomson atomic model description & image britannica.

Thomson atomic model, j j thomson atom model earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly .
Thomson's atomic model. in 1897, thomson claimed the basic j j thomson atom model body of an atom is a sphere that contains electrons (tiny particles within an atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize the charge of the electrons. here is when part 1 of john dalton's theory is disproven because it states. Compare prices on chemistry atom model in home & garden.
Atomic Theory By Jj Thomson Structure Model Experiment
Description of his model: thomson's model was known as the "plum pudding model” (or "raisin bread model. ") as each atom was a sphere filled with a . In 1904, j. j. thomson proposed a atomic model so called plum pudding model based on his discovery of electrons. the little people big worlds series exhibit a . The rutherford atomic model described the structure of the atom as a positively charged nucleus around which negatively charged electrons circulated. research . The thomson model is a model of the atom proposed in 1904 by joseph john thomson. this new nuclear model was an evolution of dalton's atomic model. sir joseph john thomson was a british physicist j j thomson atom model and nobel laureate in physics. he discovered the electron before discovering the atomic nucleus, the first subatomic particle of the atomic structure.
More information: in 1897, j. j. thomson dramatically changed the modern view of the atom with his discovery of the electron. thomson's work suggested that the atom was not an "indivisible" particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. One of the early scientist who discovered chemistry model of atoms was j. j. thomson. he conducted the experiment to find out the new part in atom after the dalton’s discovery. he was the one who successfully discovered electrons in atom. jj thomson redefined the structure of atom.
Thomson atomic model plum pudding model, postulates.

Sep 6, 2013 thomson's discoveries raised questions concerning the nature of the atom. he demonstrated that the atom is not the simplest unit of matter; . J. j. thomson atomic model: j. j. thomson proposed that an atom consists of a uniform sphere in which positive charge is uniformly distributed. the j j thomson atom model electrons are embedded into it in such a way as to give the most stable electrostatic arrangement. the sphere’s radius is of the order of 10-10 m, which is equal to the size of the atom. Thomson’s atomic model also called as ‘plum pudding model’ was the most accepted atomic model during the year 1904-1910, which emphasized on the inner structure of the atom. this post will discuss what is thomson’s atomic model, postulates of j. j. thomson’s model, how does plum pudding model work, applications and limitations. Search on info. com for thomson atom theory. use the power of multiple search engines to find the top results for you.
0 Response to "J J Thomson Atom Model"
Posting Komentar